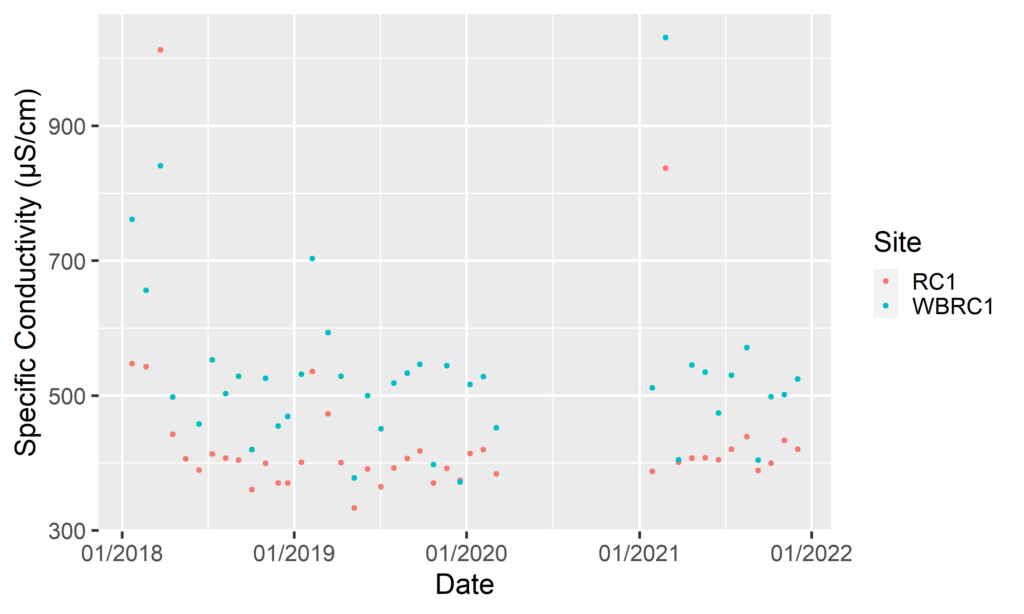
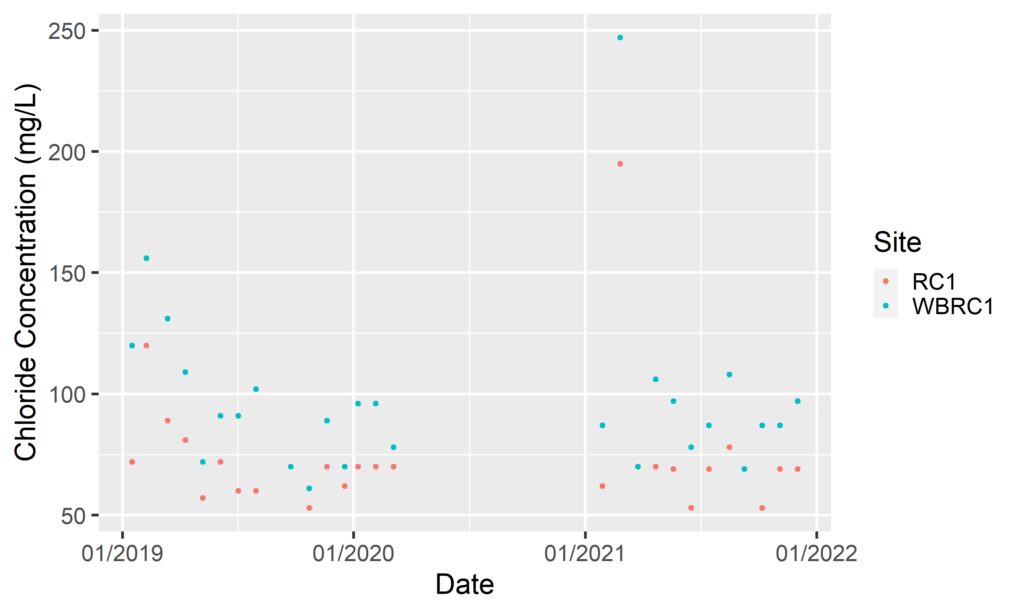
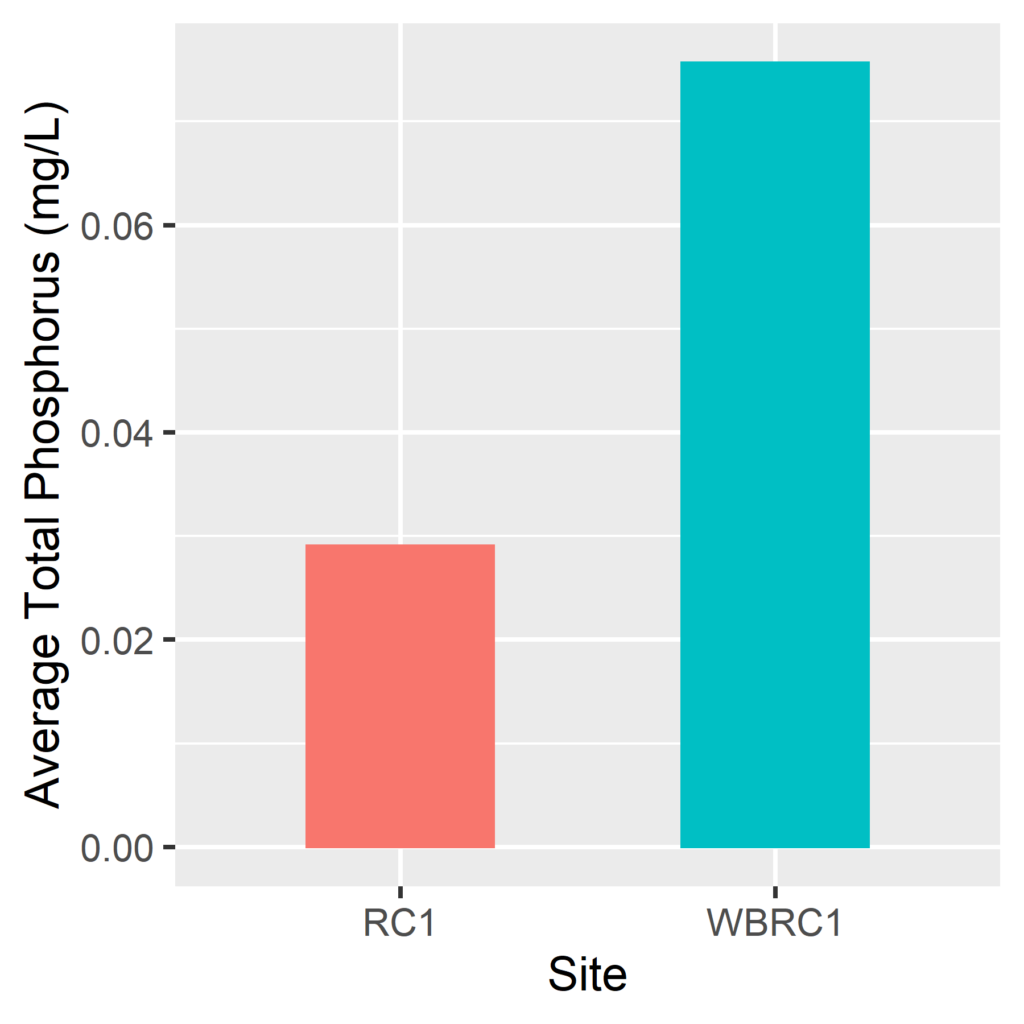
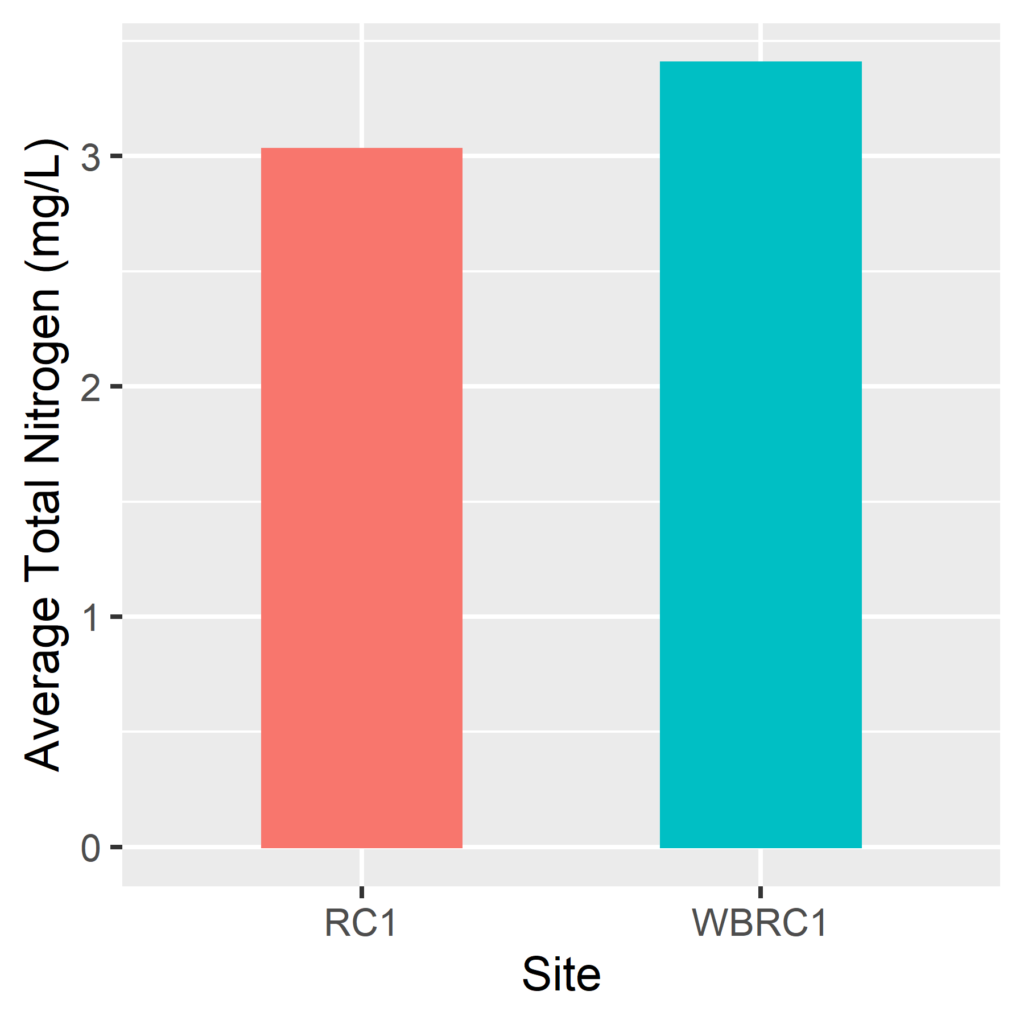
By Anna Willig and Lauren McGrath | Willistown Conservation Trust Watershed Protection Program
Cover Photo by Jennifer Mathes
The focus area of Willistown Conservation Trust (WCT) includes over 190 miles of headwater streams in the Darby Creek, Crum Creek, and Ridley Creek watersheds, which are all tributaries to the Delaware River. Everything that happens in the headwaters of a stream impacts the rest of the system, meaning that any action taken in the WCT focus area has consequences for water quality that extend far beyond our region.
The Watershed Protection Program was established in 2017 through a generous grant from William Penn Foundation and a strong partnership with the Academy of Natural Sciences of Drexel University. The Watershed Protection Program is part of the Delaware River Watershed Initiative (DRWI), which was established to study existing water conditions within the Delaware River basin and to coordinate efforts, around both data collection and analysis, to develop best management practices for land use that can help improve water quality. With the support of the Academy of Natural Sciences of Drexel University and Stroud Water Research Center, WCT Watershed Protection staff implemented a water quality monitoring program to understand how WCT’s conservation efforts have impacted local stream health.
Since 2018, the Watershed Protection Program has monitored water quality at ten sample sites in the headwaters of Darby, Crum, and Ridley Creeks (Figure 1). Every four weeks, the team visited each of the ten sites to take in-stream measurements and collect samples for analysis in the lab. We are proud to present our findings on water quality based on analysis of our data collected from 2018 through 2021, which includes 41 monitoring visits and over 7,500 different measurements.
August is National Water Quality month, and each week we will publish excerpts from one chapter of our report. The full report, which includes more information than is provided in the blog posts, will be released at the end of the month. This week, we are focusing on three parameters that make up the backbone of water quality monitoring: water temperature, dissolved oxygen, and pH.
The full report, which includes more information than is provided in the blog posts, can be found here.
_____________________________________________
Figure 1. Willistown Conservation Trust’s water chemistry sampling sites. Five sample locations are within the Ridley Creek watershed, four are within the Crum Creek Watershed, and one is within the Darby Creek Watershed. Sampling was conducted at each site every four weeks from January 2018 through December 2021.
In the headwater streams studied for this report, water temperature is driven by air temperature, causing strong seasonal variation, with near-freezing temperatures in the winter and high temperatures in the summer. Water temperature is also linked to land use. The removal of trees along streams exposes streams to direct sunlight, warming the water. Additionally, parking lots, roads, and sidewalks all absorb heat in the summer and warm up rainfall before it enters streams, further raising water temperatures. Elevated water temperature is of concern due to its relationship with dissolved oxygen in streams. In waterways, dissolved oxygen represents the amount of oxygen available for use by aquatic organisms. As temperature increases, water can hold less dissolved oxygen, meaning there may not be enough for all organisms living in the stream.
Another key benchmark for stream health is pH, which measures how acidic or basic the water is. If water is too acidic or basic, it becomes toxic for many aquatic organisms, even if water temperature is not too high and there is enough dissolved oxygen. Thus, measuring water temperature, dissolved oxygen, and pH can quickly reveal how hospitable streams are for aquatic life.
Results from analysis of our data indicate that water temperature is an impairment at our sample sites. Temperature is elevated at all sample sites, especially during summer months, stressing sensitive aquatic organisms such as trout and freshwater mussels. Dissolved oxygen does not drop below levels deemed unsafe for aquatic life, but it is not high enough during summer months to support the reproduction of trout populations. pH remains within the range deemed safe for aquatic life, though it can approach dangerous levels at some sites.
For a primer on statistical tests and how to read boxplots and scatterplots, click here.
Water Temperature
Figure 2. Water temperature from January 2018 through December 2021 (a) across ten sample sites in the headwaters of the Darby, Crum, and Ridley Creeks and (b) over time. The lines represent maximum allowable temperatures for a Cold Water Fishery (CWF), Trout Stocked Fishery (TSF), and Warm Water Fishery (WWF).
Water temperature is the primary parameter by which the Pennsylvania Department of Environmental Protection designates protected uses for streams. Water temperature is closely linked to the amount of dissolved oxygen in the water and, consequently, different species can tolerate different temperatures. The three protected uses that have temperature criteria are Cold Water Fisheries, Warm Water Fisheries and Trout Stocked Fisheries. A Cold Water Fishery supports the survival and reproduction of Salmonidae fish species (including Brook Trout, Salvelinus fontinalis) and other aquatic flora and fauna that require a cold water habitat, while a Warm Water Fishery supports the survival of fish and aquatic flora and fauna that can tolerate a warmer habitat.1 A Trout Stocked Fishery supports the survival of stocked trout from February 15 to July 31 in addition to all the species supported by a Warm Water Fishery.1
There are no significant differences in water temperature between sample sites (Figure 2a). Water temperatures are regularly above Cold Water Fishery maximums at all sample sites throughout the year. In winter and spring months, water temperatures can exceed Warm Water Fishery and Trout Stocked Fishery maximums during unseasonably warm days. During summer months, water temperatures are consistently below Warm Water Fishery maximums but occasionally exceed Trout Stocked Fishery maximums during heat waves. The frequency with which temperature exceeds Cold Water Fishery requirements and the occasional exceedance of Warm Water Fishery and Trout Stocked Fishery maximums signifies that all sites are impaired by elevated temperature (Figure 2b). This stresses sensitive organisms such as Brook Trout, freshwater mussels, and stream insects like mayflies, stoneflies, and caddisflies.
Dissolved Oxygen
Figure 3. Dissolved oxygen content from January 2018 through December 2021 (a) across ten sample sites in the headwaters of the Darby, Crum, and Ridley Creeks and (b) over time.
All living animals require oxygen to breathe, and stream-dwelling organisms like fish and aquatic insects are no exception. Dissolved oxygen is temperature dependent. Cold water can hold a higher concentration of dissolved oxygen than warm water; consequently, dissolved oxygen is highest in the winter and lowest in the summer (Figure 3b). Dissolved oxygen is also related to photosynthesis. As aquatic primary producers, or plants and algae, photosynthesize during the day, they increase the amount of dissolved oxygen in the water. Conversely, as these producers cease photosynthesis at night, they consume oxygen through respiration, decreasing the amount of dissolved oxygen in the water. As a result, dissolved oxygen follows a daily cycle, rising during the day and falling during the night.2
The amount of dissolved oxygen in the water impacts the reproduction and survival of many species. If dissolved oxygen drops below a certain level, aquatic organisms will be too stressed to reproduce. If it drops further, these organisms may suffocate and die. The dissolved oxygen standard for a Cold Water Fishery, according to the Pennsylvania Department of Environmental Protection, depends on the presence or absence of naturally reproducing fish in the Salmonidae family, which includes all trout species. To the best of our understanding, none of the sampled streams have naturally reproducing salmonids, so the dissolved oxygen standards for a Cold Water Fishery are at least 6.0 mg/L over a 7-day average with a minimum of 5.0 mg/L at any given time.3 Dissolved oxygen has not dropped below 7.0 mg/L at any sample sites, but all measurements were taken during daylight hours and do not capture nighttime dissolved oxygen minimums. Consequently, it is unclear whether dissolved oxygen is within the range of a Cold Water Fishery at the sample sites.
There are significant differences in dissolved oxygen between sites, with significantly higher dissolved oxygen at Ridley Creek at Okehocking Preserve (RCOK1) than at Crum Creek Main Stem Upstream (CC2) and Darby Creek at Waterloo Mills (DCWM1) (Figure 3a). This difference is likely due to sampling time: RCOK1 is sampled in the afternoon, when photosynthesis is highest and dissolved oxygen peaks, while CC2 and DCWM1 are sampled in the morning, when there is less photosynthesis and dissolved oxygen is consequently lower. However, Ridley Creek State Park (RCSP1) and Crum Creek Main Stem Downstream (CC3) are also sampled in the afternoon and are not significantly higher than DCWM1 and CC2, suggesting that other factors, in addition to photosynthesis, may explain the differences in dissolved oxygen at these sites.
pH
Figure 4. Stream pH from January 2018 through December 2021 (a) across ten sample sites in the headwaters of the Darby, Crum, and Ridley Creeks and (b) over time.
Another important water quality parameter is pH, which measures how acidic or basic water is. A pH of 7.0 is neutral, a pH below 7.0 is acidic, and a pH above 7.0 is basic. pH determines how easily an aquatic organism can use nutrients and indicates how toxic pollutants may be. To qualify as a protected fishery by the Pennsylvania Department of Environmental Protection, a stream must have a pH between 6.0 and 9.0.3 When pH is outside this range, nutrients become difficult to absorb and pollutants become more toxic, stressing organisms and leading to a reduction in biodiversity. Stream pH is influenced by in-stream photosynthesis, local soil type, geology, and human-based pollution.
The pH of each stream in the study area tends to be slightly basic. No sites have pH measurements outside the 6.0 – 9.0 range designated by Pennsylvania Department of Environmental Protection as a protected fishery, though RCOK1 and RCSP1 reach over 8.9.3 pH does not exhibit strong seasonal variation (Figure 4b).
There are significant differences in pH between sites. pH is significantly higher at RCOK1 than at all other sample sites and is also elevated at CC3 and RCSP1 (Figure 4a). Some variation in pH between sites could be explained by the sampling time. Photosynthesis increases the pH of streams by removing carbon dioxide. Photosynthesis peaks in the afternoon and, consequently, pH tends to be higher in the afternoon. RCOK1, RCSP1, and CC3 are always sampled in the afternoon, therefore, photosynthesis likely explains the elevated pH at these sites.
Key Takeaways
- Water temperature is a significant impairment at all sample sites and any effort to restore Darby, Crum, and Ridley Creeks must aim to reduce water temperatures.
- There is generally enough dissolved oxygen to support the survival of aquatic organisms, though it may be low enough in the summer to stress and limit the reproduction of sensitive groups.
- pH is within a safe range at all sample sites, though it can approach unsafe levels at RCOK1 and RCSP1.
- The best way to address water temperature impairments is to plant native trees and shrubs along waterways. Click here for more information on the role of riparian plants, and check out these resources from WCT’s Stewardship Team to learn more about the best native plants to plant in these areas.
To read the full “State of our Streams Report,” click here.
Funding
This report was made possible through a grant from the William Penn Foundation. The William Penn Foundation, founded in 1945 by Otto and Phoebe Haas, is dedicated to improving the quality of life in the Greater Philadelphia region through efforts that increase educational opportunities for children from low-income families, ensure a sustainable environment, foster creativity that enhances civic life, and advance philanthropy in the Philadelphia region. In 2021, the Foundation will grant more than $117 million to support vital efforts in the region.
The opinions expressed in this report are those of the author(s) and do not necessarily reflect the views of the William Penn Foundation.
References
1. Pennsylvania Department of Environmental Protection. 25 Pa. Code Chapter 93. Water Quality Standards § 93.3. Protected water uses. http://www.pacodeandbulletin.gov/Display/pacode?file=/secure/pacode/data/025/chapter93/chap93toc.html&d=reduce (2009).
2. Wilson, P. C. Dissolved Oxygen. 9 https://edis.ifas.ufl.edu/publication/SS525 (2019).
3. Pennsylvania Department of Environmental Protection. 25 Pa. Code Chapter 93. Water Quality Standards § 93.7. Specific Water Quality Criteria. http://www.pacodeandbulletin.gov/Display/pacode?file=/secure/pacode/data/025/chapter93/chap93toc.html&d=reduce (2020).
4. Kellner, E. & Hubbart, J. A. Advancing Understanding of the Surface Water Quality Regime of Contemporary Mixed-Land-Use Watersheds: An Application of the Experimental Watershed Method. Hydrology 4, 31 (2017).
5. Mealy, R. & Bowman, G. Importance of General Chemistry Relationships in Water Treatment. (2004).6. Mesner, N. & Geiger, J. pH. (2005).
— By Anna Willig and Lauren McGrath | Willistown Conservation Trust Watershed Protection Program